Miscelánea
water and landscape
AGUA y TERRITORIO
Systematic literature review on agrochemicals impacts dormant eggs in temporary wetlands: an ocean of unknowingness
Revisión sistemática sobre los impactos de los agroquímicos en los huevos de resistencia en humedales temporales: un océano de desconocimiento
Gema Parra
Department of Animal Biology, Plant Biology and Ecology University of Jaén
Jaén, Spain
 ORCID: 0000-0002-4519-4799
ORCID: 0000-0002-4519-4799
Información del artículo
Recibido: 3 febrero 2022
Revisado: 17 mayo 2022
Aceptado: 13 junio 2022
ISSN2340-8472
ISSNe2340-7743
 CC-BY
CC-BY
© Universidad de Jaén (España).
Seminario Permanente Agua, Territorio y Medio Ambiente (CSIC)
ABSTRACT
Temporary wetlands are recognised biodiversity hotspots. Dormant egg banks, as part of their cryptic biodiversity, are responsible of wetlands resilience. Egg banks are also known to be sensitive indicators of anthropogenic disturbances. This study aims to assess the current state of research of agrochemical impact on dormant egg banks in temporary wetlands. The systematic literature review carried out has shown the small number of studies on this topic. This study provides evidence of commonality concerning negative impact effect on the organisms, reducing hatching success, dormant eggs production and emergence, or species richness, among others, which might weaken ecosystem stabilization mechanisms by reducing biodiversity. Our review also revealed a glaring lack of in situ and long-term studies for understanding ecosystem consequences of toxicants on temporary wetlands. These gaps in knowledge hamper our ability to design and implement evidence-based conservation and management programs but opens opportunities for new research lines.
KEYWORDS: Egg bank, Recovery, Resilience, Systematic review, Temporary ponds.
RESUMEN
Los humedales temporales atesoran una alta biodiversidad. Los bancos de huevos de resistencia, como parte de su biodiversidad críptica, responsables de la resiliencia también son indicadores sensibles de perturbaciones antropogénicas. Este estudio pretende evaluar el grado de conocimiento sobre el impacto de los agroquímicos en los huevos de resistencia en humedales temporales. La revisión sistemática de la literatura realizada ha puesto de manifiesto el escaso número de estudios sobre este tema. La mayoría indican un impacto negativo: menor éxito de eclosión, menor producción y emergencia, o menor riqueza de especies, lo que debilita el ecosistema al reducir la biodiversidad. Además, hay una evidente falta de estudios in situ y a largo plazo para comprender las consecuencias de estos tóxicos en los humedales temporales. Esto dificulta nuestra capacidad para diseñar e implementar programas de conservación y manejo basados en la evidencia científica, pero abren oportunidades para nuevas líneas de investigación.
PALABRAS CLAVE: Banco de huevos, Humedales temporales, Revisión sistemática, Recuperación, Resiliencia.
Revisão sistemática da literatura sobre impactos de agroquímicos em ovos dormentes em áreas úmidas temporárias: um oceano de incognitas
RESUMO
As zonas úmidas temporárias são sistemas que armazenam alta biodiversidade. Bancos de ovos resistentes, como parte de sua biodiversidade críptica, responsáveis pela resiliência, também são indicadores sensíveis de perturbações antrópicas. Este estudo tem como objetivo avaliar o grau de conhecimento sobre o impacto de agroquímicos em ovos resistentes em áreas úmidas temporárias. A revisão sistemática da literatura realizada revelou o pequeno número de estudos sobre o assunto. A maioria indica um impacto negativo: menor sucesso de eclosão, menor produção e emergência, ou menor riqueza de espécies, o que enfraquece o ecossistema ao reduzir a biodiversidade. Este estudo também mostra que há uma evidente falta de estudos in situ e de longo prazo para entender as consequências desses tóxicos em áreas úmidas temporárias. Isso dificulta nossa capacidade de projetar e implementar programas de conservação e manejo baseados em evidências científicas, mas abre oportunidades para novas linhas de pesquisa.
PALAVRAS-CHAVE: Banco de ovos, Zonas húmidas temporárias, Revisão sistemática, Recuperação, Resiliência.
Revisione sistematica della letteratura sugli impatti dei prodotti fitosanitari sulle uova dormienti nelle zone umide temporanee: un oceano di incognite
SOMMARIO
Le zone umide temporanee sono sistemi che immagazzinano un’elevata biodiversità. I banchi di uova di resistenza, come parte della loro criptica biodiversità, responsabile della resilienza, sono anche indicatori sensibili di disturbo antropico. Questo studio mira a valutare il grado di conoscenza dell'impatto dei prodotti chimici per l'agricoltura sulle uova di resistenza nelle zone umide temporanee. La revisione sistematica della letteratura effettuata ha messo in luce l'esiguo numero di studi sull'argomento. La maggior parte indica un impatto negativo: minore successo della schiusa, minore produzione ed emergenza o minore ricchezza di specie, che indebolisce l'ecosistema riducendo la biodiversità. Questo studio mostra anche che c'è una cospicua mancanza di studi in situ ea lungo termine per comprendere le conseguenze di questi tossici nelle zone umide temporanee. Ciò ostacola la nostra capacità di progettare e attuare programmi di conservazione e gestione basati su prove scientifiche, ma apre opportunità per nuove linee di ricerca.
PAROLE CHIAVE: Banca delle uova, Zone umide temporanee, Revisione sistematica, Recupero, Resilienza.
Une revue systématique de la littérature sur les impacts des produits agrochimiques sur les œufs dormants dans les zones humides temporaires: un océan d'ignorance
RÉSUMÉ
Les zones humides temporaires ont une grande biodiversité. Les bancs d'œufs de résistance, dans le cadre de leur biodiversité cryptique, sont également des indicateurs sensibles de perturbation anthropique. Cette étude vise à évaluer le degré de connaissance de l'impact des produits agrochimiques sur les œufs de résistance dans les zones humides temporaires. La revue systématique de la littérature réalisée a révélé le faible nombre d'études sur ce sujet, avec impact négatif : moindre succès d'éclosion, moindre production et émergence, ou moindre richesse spécifique, ce qui fragilise l'écosystème en réduisant la biodiversité. Par ailleurs, il existe un manque d'études in situ et à long terme pour comprendre les conséquences de ces toxiques dans les zones humides temporaires. Cela entrave notre capacité à concevoir et à mettre en œuvre des programmes de conservation et de gestion basés sur des preuves scientifiques, mais ouvre des opportunités pour de nouvelles voies de recherche.
MOTS-CLÉS: Banque d'œufs, Zones humides temporaires, Revue systématique, Récupération, Résilience.
Introduction
Temporary wetlands, with distinctive wet-dry cycles, typify the aquatic environment in dryland areas of the world and elsewhere1. They host an array of unique plant and animal species that are highly adapted to the biophysical settings and disturbance regime of temporary ponds and not found in permanent aquatic habitats2. More generally, temporary wetlands are recognised as biodiversity hotspots3, and its cryptic component, comprised of microscopic plants and animals invisible to the naked eye that collectively form seed and resting egg banks, are especially valuable from ecological, evolutionary and applied perspectives4. Zooplankton dormant egg banks, for instance, preserve an important component of genetic diversity5, and contribute through the storage effect and bet-hedging strategies to safeguard ecological processes in these highly unpredictable environments6.
Despite their uniqueness, temporary wetlands are among the most threatened ecosystem types by land use and climate change, aggravated by the general low public awareness of these ecosystems7 or lack of consideration in policy, such as the European Water Framework Directive8. Dormant egg banks have been suggested to be vulnerable to the disruptions arising from natural and anthropogenic perturbations and therefore useful indicators of ecological stress and impact assessment9. Assessing the emergence process when animals colonize the water column upon rewetting of the sediment may detect changes in species dominance and community composition arising from environmental impact10. Such disruptions may occur either directly through toxicity-induced lethality or indirectly through deterioration of water quality11, incurring fitness costs in animals12.
Emissions of toxic substances have reached levels that exceed planetary boundaries, contributing to an erosion of the resilience in major components of Earth-system functioning13. There is special concern regarding agrochemicals because these toxicants are released into aquatic systems at unprecedented rates worldwide14. Specifically, intensive agriculture poses a major threat to wetlands due to sediment accumulation resulting from ploughing watersheds and point and non-point sources of agrochemical pollution disrupting their biophysical environment15. It is well known that toxic substances cause changes in active plankton communities, create imbalances in food webs and decrease water quality16. Moreover, climate change forecasts high evaporation in drylands, which might lead to higher concentrations of toxicants in wetlands17. Ultimately, this has consequences in the resilience and the recovery capacity from agricultural impact in temporary wetlands18.
Resilience theory has gained a strong foothold in the recent environmental sustainability literature as it accounts not only for the ability of ecosystems, and other complex systems of people and nature, to adapt to, but also to transform in response to anthropogenic impact19. Resilience theory embraces both the ability of a system to withstand (“robustness”) and recover from stress. In temporary wetlands, recovery is mediated by a combination of internal (buffering capacity of egg banks) and external (dispersal) factors20. Resilience theory also recognizes the ability of ecosystems to undergo fundamental change when disturbance thresholds are exceeded and shifts between alternative system regimes occur21. Such changes are especially pervasive in agricultural landscapes, where shallow lakes shifting from clear-water, macrophyte dominated regimes to turbid regimes dominated by phytoplankton due to nutrient enrichment attest to agriculture’s profound alteration of the natural environment22. Although, recovery and robustness are prevailing views of resilience in temporary wetland science and management, there is preliminary evidence that also temporary ponds can undergo regime shifts23, often as a result of excessive nutrient enrichment that are phenomenological similar to those observed in shallow lakes24. Accounting for different aspects of resilience, such as recovery capacity, or alternative regimes achievement, is therefore crucial for effective management and conservation of natural resources25.
To assess the current state of knowledge about the impacts of toxicants on temporary wetland egg banks and their resilience, this study provides a systematic literature review. Systematic literature reviews have been introduced to the ecological literature for their potential to reduce bias and reveal a more accurate picture of the state of knowledge26. Given the relevance of temporary wetland egg banks for basic and applied ecological research, this study aims to assess the current state of research of toxicants impact on dormant egg banks. Specifically, the review and synthesize approaches used for inferring impact and discuss the implications for resilience that can be derived from them. Ultimately, the review shall identify critical knowledge gaps that may hamper the effective management and conservation of temporary ponds on a fast-changing planet.
Material and methods
A systematic literature review was carried out with the goal of reducing bias by identifying, appraising, and synthesizing all relevant studies on toxicants impact on egg banks of temporary wetlands published in the peer-reviewed literature. A systematic review is a research synthesis on a precisely defined topic using explicit and strict protocols to identify, select, appraise, and analyse references27. Pullin and Steward28 devised a set of guidelines for undertaking a formalized systematic literature review, including search strategy, protocol formation, data inclusion, data extraction, and analysis. In the present study the protocol consisted of the following steps: a) defining the research question: how does the effect of toxicants influence dormant eggs and therefore the capacity of temporary wetlands to recover from their effects? b) defining criteria for the inclusion and exclusion of studies in the review (language, type of ecosystem, type of organism, type of document, knowledge discipline); c) implement the search strategy; d) data extraction (type of study, studied organism/community, substance, selected endpoint, future consequences) and e) analysis of the information.
The literature search for the systematic review was carried out in Scopus. This bibliographic database has several advantages, such as ease of navigation, includes 100% of what is indexed other databases, facilitates access to cited documents and is accessible through Internet, among others29. Considering our research question, the following search string was used: topic = “ecotoxicology” AND topic = (“dormant egg” OR “diapausing egg” OR “resting egg”). In a second search process the topic = (“fertilizer” OR “pesticide” OR “herbicide” OR “fungicide”) was added. Finally, the last topic included in the process was “wetland”.
Next, the “refine” function in Scopus was use to exclude articles other languages (Chinese), as well as time period selected (published before 2001). The screening of the titles and abstracts was used to determine whether studies should be excluded. Reviews and book chapters and books were excluded, as the study focus on primary information sources, to extract the specific information used in the analysis. Papers concerning other ecosystems (i.e.: marine) or other organisms (i.e.: vegetation, fish) were not included in the analysis.
The information analysis on the selected papers was conducted on the full-text articles, extracting the following data from the papers: type of study (lab indoor study, mesocosm study, outdoor field study), studied organisms (single species studies, multi-species studies), toxic substance (type of toxic, single toxicant or multiple toxicants studies), exposure period (in days), chemical (heavy metals, pesticides, herbicides, etc.), endpoint of biological hierarchical level (individual, population, community) and hypothesized ecosystem consequences (resistance, resilience, recovery).
The direction of the effect in the studies (negative or positive) was analysed using a non-parametric test. The sign test was used to demonstrate that the number of studies with a determined result (for example, negative impact of agrochemical on egg emergence) is significantly greater than the opposite result; that is, it is greater than that expected by chance30. It is based on binomial distribution31 and the p value for the test indicates the probability of error in rejecting the null hypothesis of equal effects.
Results
The first search step initially identified 622 studies; the list was reduced to 270 with the second search step, and finally 60 studies were identified with all the searching terms selected. To include papers that matched the search criteria after refining the search the “refine” function in Scopus was used (language 1; publishing date 1; books 6; book chapter 3, reviews 8). Additionally, the title and the abstract screening allow removing 24 articles that were unrelated to the study goal. Finally, only 14 papers from the initial set matched the criteria (Figure 1).
Figure 1. Flow chart of the systematic literature review process and inclusion/exclusion criteria
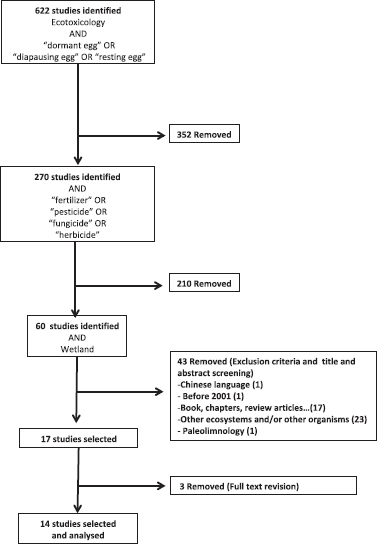
Source: own elaboration.
Table 1 summarises the results of the selected studies about the effects of toxicants on different biological endpoints. The available evidence has highlighted the important constraints on dormant eggs and reproduction responses as a result of pollution, which, in turn, affects populations and/or communities. The sign test indicates that the number of studies that report negative impact (#13) is greater than the number of studies with no effect (#1) (p = 0.002).
Table 1. Studies found following the research string and selected information extracted
Study |
Type of study, according to… |
Exposure period (d) |
Toxicant |
Endpoints Hierarchical level |
Hypothesized consequences |
||
Site |
# Toxic |
# Species |
|||||
Indoor Lab experiment |
Single toxicant |
Multiple species (several rotifer and microcrustacean species) |
14 |
Glyphosate |
Community (taxa richness, abundance and Shannon index of the emerged zooplankton community) |
Significant reduction in rotifer taxon richness and emergence of viable resting eggs after exposition, which leads to rapid community recovery |
|
Indoor Lab experiment |
Multiple toxicant (no mixture) |
Single species (Moina macrocopa) |
240 |
Heavy metals (copper, cadmium, zinc and nickel) |
Population (resting egg emergency) |
High resistance of resting eggs to high concentrations of heavy metals over a relatively long time. Life tables showed no effects. No consequences in recovery |
|
Indoor Lab experiment |
Single toxicant |
Multiple species (Brachionus plicatilis and B. quadridentatus) |
30 |
Copper |
Population (growth rates, and resting eggs production) |
Reduced niche overlap between coexisting species to minimize the impact of fitness inequalities on competitive interactions, allowing stabilized coexist |
|
Indoor Lab experiment |
Single toxicant |
Single species (Daphnia magna) |
14 (short) 56 (long) |
Fenoxycarb |
Population (juvenile production, ephippia production and hatching rate) |
Effects on the size and buffering capacity of the dormant egg bank, potentially have an effect on recovery and resilience of aquatic ecosystems |
|
Indoor Lab experiment |
Multiple toxicant (mixture) |
Multiple species (34 taxa in zooplankton community) |
28 |
Herbicides based on 2,4-D and glyphosate mixture |
Community (abundance and taxon richness of the emerged zooplankton community) |
Changes in community composition. Increased risk in local extinction |
|
Indoor Lab experiment |
Multiple toxicant (no mixture) |
Single species (Brachionus plicatilis) |
2 (short) 21 (long) |
Copper, zinc, aluminium and arsenic |
Population (survival, hatching success, population growth rate) |
Alteration of the structure and diversity of egg banks, induced selection of resistant phenotypes, impact on the genetic variability of populations. Modification on structure and function of diapausing egg bank |
|
Indoor Lab experiment |
Single toxicant |
Multiple species (30 taxa in zooplankton community) |
30 |
Glyphosate |
Community (richness, abundance and evenness Population (time of the first hatching, frequency of hatching) |
The sensitivity of resting stages to some specific environmental stressors (such as pesticides) may be playing a role in such recovery hampering |
|
Outdoor Mesocosm experiment |
Single toxicant |
Multiple species (Daphnia pulex, Lithobates pipiens, phytoplankton and peryphyton) |
83 |
Chlorpyrifos |
Population (cladoceran survival and population growth rate, amphibian survival and growth rate) |
The entire food web was affected by simply altering the population of D. pulex (indirect effects). Trophic cascade events diminished where tolerant populations |
|
Indoor Lab experiment |
Multiple toxicant (mixture) |
Multiple species (Daphnia longispina complex) |
15 |
Irgarol, triclocarban, benzotriazole, 5-methylbenzotriazole, octocrylene, propiconazole, terbutryn, prochloraz, triclosan |
Population (hatching success, mortality) |
May interfere with bet-hedging strategies, lead to a depletion of the egg bank, and thus increase the extinction risk of local populations. |
|
Indoor Lab experiment |
Single toxicant |
Single species (Daphnia magna) |
10 |
Fenoxycarb |
Population (development, hatching success, timing of hatching, Egg’s Fenoxycarb concentration |
Impact the size and structure of zooplankton dormant egg banks. Small active population sizes could further lower the amount of produced dormant stages, eroding the buffering capacity of the egg bank against the risk of local extinction and loss of genetic diversity |
|
Indoor Lab experiment |
Single toxicant |
Multiple species (Simocephalus vetulus and Daphnia pulex) |
2 |
Chlorpyrifos |
Population (survival) |
Population-level variation in pesticide resistance may have community-wide consequences, buffering cascade effects |
|
Indoor Lab experiment |
Multiple toxicant (no mixture) |
Single species (Daphnia magna) |
10 |
Fenoxycarb and carbaryl |
Population (hatching success, growth, survival) Individual (abnormalities) |
Decrease in dormant egg bank may reduce genetic variation, hence the evolutionary potential of a population |
|
Indoor Lab experiment |
Multiple toxicant (no mixture) |
Single species (Pseudosida ramosa) |
2 (short) 21 (long) |
Sodium and potassium |
Population (survival, fecundity and fertility) |
Long-term effect in populations, biota cannot adapt and freshwater taxa may become locally extinct, transferring dominance to salt-tolerant taxa |
|
Outdoor Field experiment |
Single toxicant |
Multiple species (13 taxa) |
21 (short) 365 (long) |
Rotenone |
Community (abundance and richness) |
Zooplankton community recovery rate vary considerably. Application time management strategies would facilitate system recovery |
|
Source: own elaboration.
The results specifically show that a large number of studies (n = 12) have been carried out in laboratory settings, testing for a single toxic compound (n = 8) or multiple toxicants (n = 6), either individually or in mixtures. Toxicity was tested for heavy metals (n = 3) and organic chemical compounds (n = 9), most of them used as insecticides, herbicides or/and fungicides. Biological endpoints chosen were either single populations of rotifers and daphnids (n = 6) or multiple taxa (n = 6; ranging from 2 to 34 spp.). These studies focused largely on population level aspects related to survival, hatching, growth rates, and resting egg production (n = 7), while community-based assessments (n = 4) focusing on richness, abundance, evenness and diversity were low. The exposure period ranged from 2 days to one year (Figure 2). With only two outdoor studies, one designed using mesocosm and the other using different sampled wetlands (some of them were semipermanent), research in the field was comparatively underrepresented (15%; Figure 2). These studies tested for the effects of either chlorpyrifos or rotenone on the food web, including phytoplankton, periphyton, zooplankton and an amphibian species or the zooplankton and macroinvertebrate community, respectively. Biological responses to the stressors were risk of local extinction, selection of resistant phenotypes, and changes to more tolerant communities. Inference about aspects of resilience was rather implicit and focusing on the recovery from contamination (see e.g.32). None of the reviewed studies discussed their results from the viewpoint of ecological resilience.
Figure 2. Exposure time (days) used in the selected studies. Four of them appear twice as they carried out two exposure periods (the short and the long, in bracket). Outdoors studies are represented with squares and indoors studies are represented with circles
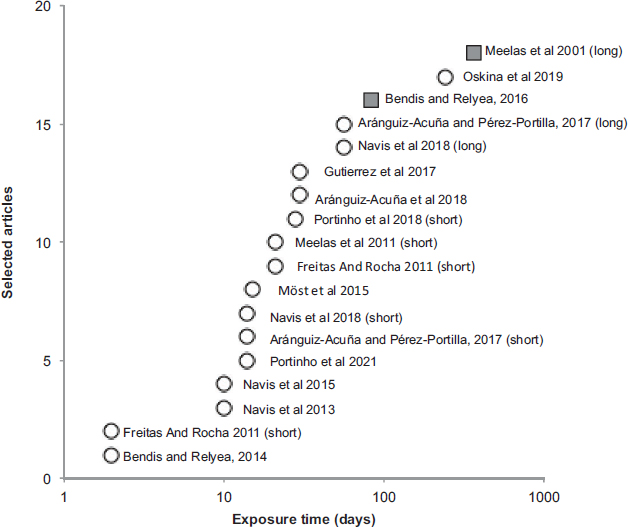
Source: own elaboration.
Discussion
Eggs banked in dry soils have attracted much research interest because they are known to be sensitive indicators of anthropogenic disturbances and thus useful for environmental impact assessment33. The findings of this study were discouraging because only a very small number of studies met the criteria for our search chain and goal. Additionally, none of the reviewed studies were actually conducted in temporary wetlands nor did they use organisms collected from temporary wetlands. Moreover, they did not assess effects directly on dormant stages. In addition to the scarcity of information, it should be noted that approaches used to assess contamination impacts varied widely across studies, suggesting the lack of a harmonized and commonly applied protocol for assessing agrochemical impacts on egg banks. Despite the variability in approaches, there was, however, agreement across the majority of studies that toxicants affect organisms negatively, reducing hatching success, dormant eggs production, or species richness, among others. Because these results are overwhelmingly based on lab studies, the implications of these findings for more ecologically realistic ecosystem conditions are at this stage speculative. However, two broad ecological response types have been discussed in the selected papers that can helps us to detect knowledge gaps and stimulate the necessary research for addressing management and sustainability challenges of temporary ponds.
The first relates to compensational processes that may buffer against the impact of toxicants. For instance, despite negative impact at the population level likely positive effects at the community level have been envisioned; for instance, through the reduction in the intensity of competition between populations, which promotes their coexistence over time34. Similarly, Bendis and Relyea35 reported that despite negative effect on some cladocerans and amphibians’ population growth rates due to chlorpyrifos exposure, cascading trophic interactions lead to stabilize food webs and might contribute to robustness of these systems against disturbance from agrochemicals.
The second relates to negative ecological consequences of toxicants in wetlands. For instance, three studies36 suggested that the negative effect detected at the community level would lead to changes in community composition with an increased risk in local extinction rates. The negative effects detected in egg bank hatching success also erodes its buffering capacity against stress from agrochemicals and increase the risk of local extinction and the loss of genetic variability37. Such effects are often mediated by the synchrony between environmental factors and lifecycles together with other biological characteristics, which influence the degree of impact and recovery38. For instance, it39 was detected sharp reductions in abundance after rotenone exposures of zooplankton communities, but nearly all affected taxa recovered by the following spring, also confirmed by Portinho et al40. Such recovery could be mediated by “temporal escape strategy”, whereby changes in hatching dynamics facilitate recovery41. It is clear that the timing of impact-response relationships needs to be accounted for revealing direct and indirect ecological effects that ensue from toxicity, trans-generational effects and altered environmental conditions42. The increase in knowledge on recovery capacity mediated by resting egg banks will allow carrying out restoration activities based on evidence43.
The aforementioned factors may operate on resilience of temporary wetlands to disturbances from toxicants. Although, resilience theory has gained center-stage in the environmental sustainability discourse, it has not melded well with the science and management of temporary wetlands. In this sense, the discussions on the reviewed papers focus largely on the ability of egg banks to either withstand or recover from stress, but this focus on robustness and recovery ignores the fact that ecosystems can exist in alternative stable regimes. This is an inherent feature in ecological resilience44; i.e., the amount of disturbance an ecosystem can absorb without changing structure, functions and feedbacks45. There is a rich body of literature documenting that shallow lake ecosystems change from a clear-water regime dominated by submerged macrophytes to a turbid regime characterized by phytoplankton blooms due to nutrient loading46. And there is evidence that excessive nutrient enrichment can also change temporary wetlands between alternative regimes similar to the shifts documented for lakes47. This preliminary evidence combined with the results of this review make clear that regime changes, in addition to recovery and robustness, need to be accounted to understand the broader consequences of pollutants on temporary wetlands resilience and devise sound management plans48.
Effective watershed management strategies are critical for protecting aquatic ecosystems affected by agricultural practices. When dry temporary ponds rewet, resting eggs receive the necessary environmental cues for initiating a new life cycle49. Rewetting therefore essentially comprises the opening of a “window of opportunity” for both, organisms to reproduce and scientists and managers to assess the ecological integrity of wetlands through the emergence process of organisms from sediments50. However, pollution events may alter the biophysical hatching environment for organisms upon rewetting, leading to a “false window of opportunity” (Figure 3). Provided that organisms maintain their ability to hatch, they may essentially emerge into an ecological trap, mired into polluted habitats less suitable for them51. The consequences of emerging into a degraded habitat may lead to reproductive failure in the long term, resulting in depauperate egg banks52.
Figure 3. Schematic framework for the depauperated egg bank and window of opportunities under toxicants exposure
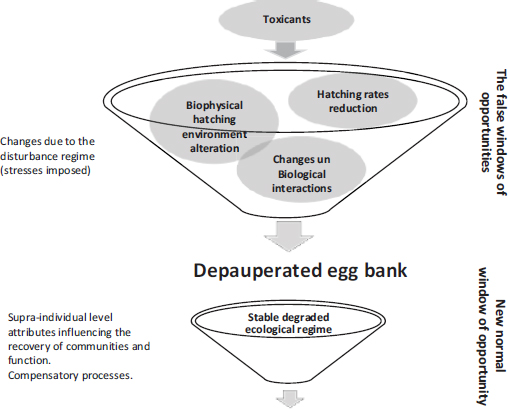
Source: own elaboration.
Management strategies can take advantages from such windows of opportunities by timing application of agrochemicals considering the periods when wetlands rewet53. That is, agrochemicals released into the ecosystem through nonpoint source pollution and the beginning of the wet period could be temporarily disconnected. Regulating safe periods when agrochemicals apply is restricted may be useful for creating windows of opportunities. For instance, uncoupling agrochemicals apply periods from periods when wetland communities establish from dormant egg bank may be useful for closing false windows of opportunities. So is likely, regulation to temporally restrict use of agrochemicals should account for half-lives (DT50 in water or water-sediment phase) of toxicants. For example, fenoxycarb and carbaryl have DT50 shorter than 1 month. Applying these products in advance of the wet season may contribute to minimizing impact. However, in the case of agrochemicals with longer DT50 (i.e., terbutryn or glyphosate) false windows of opportunity may be open during the wet season and beyond.
Given the disruptive nature of toxicants and their metabolites on ecological interactions (predation, competition) as well as the particular sensitivity of the emerged community to natural environmental changes (droughts, oxygen depletion, changes in temperature or hydroperiod54), it is recommendable to follow the precautionary principle and ban persistent agrochemicals from temporary wetland watersheds. Lahr et al.55 described a management approach advocating that agrochemical treatments should not be carried out in the vicinity of temporary ponds. He also recommended proper applying methods to avoid pollutants drift over large distances in the Sahel region (Southern edge Sahara Desert, Africa) during the desert locusts chemical control programs. Protecting temporary wetlands in dry areas, as many environmental challenges, is a complex task and there is no simple solution. Apart from scientific evidence, a collaborative approach and consensus between the different stakeholders involved is essential.
Although systematic literature reviews allow to assess current trends of research56, they also have limits due to the subjective definition of search strings and interpretation57, which is often, influence by researcher’s fields of expertise58. Thus, systematic literature reviews, like ours, have recognized limitations in terms of an absolute representation of the current-state-of-the-art of research. For instance, relevant references may be missed because they did not match the search string. The limitation is also associated to statistical approaches used, like the sign test, which neither considers statistical power nor effect size, but which is still commonly used in heterogeneous studies where aims are to compare the direction of the effects59. The strength of such reviews therefore resides in manifesting knowledge gaps that can pave the way for future research. The present review supported the idea that toxicants are detrimental to resting eggs production and emergence, but revealed a glaring lack of in situ and long-term studies for understanding ecosystem dynamics, including resilience. The extracted information makes us aware of the lack of “weight of evidence” needed to inform policy makers and manager on the toxicant long-term effect in temporary wetlands. Future research is badly needed to salvage a valuable ecosystem type that increasingly vanishes from our landscapes.
Acknowledgments
The research group RNM300, Ecology and Biodiversity of aquatic systems, University of Jaén, supported this study. I would like to thank David Angeler whose conversations and generosity during the UNIA 2020 workshop and subsequent talks made this manuscript possible.
REFERENCES
Allen, Craig R; Angeler, David G.; Chaffin, Brian C.; Twidwell, Dirac; Garmestani, Ahjond. 2019: “Resilience reconciled”. Nature Sustainability, 2, 898–900. https://doi.org/10.1038/s41893-019-0401-4
Angeler, David G. 2007: “Resurrection ecology and global climate change research in freshwater ecosystems”. Journal of the North American Benthological Society, 26, 12–22. https://doi.org/10.1899/0887-3593(2007)26[12:REAGCC]2.0.CO;2
Angeler, David G. 2021: “Conceptualizing resilience in temporary wetlands”. Inland Waters, 11, 467–475. https://doi.org/10.1080/20442041.2021.1893099
Angeler, David G.; García Gregorio. 2005: “Using emergence from soil propagule banks as indicators of ecological integrity in wetlands, advantages and limitations”. Journal of the North American Benthological Society, 24(4), 740–752. https://doi.org/10.1899/05-025.1
Angeler, David G.; Moreno, José M. 2006: “Impact-recovery patterns of water quality in temporary wetlands after fire retardant pollution”. Canadian Journal of Fisheries and Aquatic Sciences, 63(7), 1617–1626. https://doi.org/10.1139/f06-062
Angeler, David G.; Moreno, José M. 2007: “Zooplankton community resilience after press-type anthropogenic stress in temporary ponds”. Ecological Applications, 17(4), 1105–1115. https://doi.org/10.1890/06-1040
Ansari, Abid A.; Singh, Gill Sarvajeet; Lanza, Guy R.; Rast, Walter (Eds.). 2011: Eutrophication: causes, consequences and control (vol. 1). Dordrecht (Holland), Springer Science Business Media. https://doi.org/10.1007/978-90-481-9625-8
Aránguiz-Acuña, Adriana; Serra, Manuel. 2016: “Diapause as escape strategy to exposure to toxicants, Response of Brachionus calyciforus to arsenic”. Ecotoxicology, 25(4), 708–719. https://doi.org/10.1007/s10646-016-1629-7
Aránguiz-Acuña, Adriana; Pérez-Portilla, Pablo 2017: “Metal stress in zooplankton diapause production, post-hatching response”. Ecotoxicology, 26, 329–339. https://doi.org/10.1007/s10646-017-1766-7
Aránguiz-Acuña, Adriana; Pérez-Portilla, Pablo; De la Fuente, Ana; Fontaneto, Diego. 2018: “Life-history strategies in zooplankton promote coexistence of competitors in extreme environments with high metal content”. Scientific Report, 8. https://doi.org/10.1038/s41598-018-29487-3
Baho, Didier L.; Allen, Craig R.; Garmestani, Ahjond; Fried-Petersen, Hannah B.; Renes, Sophia E.; Gunderson, Lance. H.; Angeler, David G. 2017: “A quantitative framework for assessing ecological resilience”. Ecology and Society, 22(3), 17. https://doi.org/10.5751/ES-09427-220317
Bendis, Randall J.; Relyea, Rick A. 2014: “Living on the edge, Populations of two zooplankton species living closer to agricultural fields are more resistant to a common insecticide”. Environmental Toxicology and Chemistry, 33, 2835–2841. https://doi.org/10.1002/etc.2749
Bendis, Randall J.; Relyea, Rick A. 2016; “Wetland defence, naturally occurring pesticide resistance in zooplankton populations protects the stability of aquatic communities”. Oecologia, 181, 487–498. https://doi.org/10.1007/s00442-016-3574-9
Brendonck, Luc; De Meester, Luc. 2003: “Egg banks in freshwater zooplankton, Evolutionary and ecological archives in the sediment”. Hydrobiologia, 491(1-3), 65–84. https://doi.org/10.1023/A:1024454905119
Burnham, Judy F. 2006: “Scopus database: a review”. Biomedical digital libraries, 3(1), 1. https://doi.org/10.1186/1742-5581-3-1
Cáceres, Carla. E. 1997: “Dormancy in invertebrates”. Invertebrate Biology, 116, 371–383. https://doi.org/10.2307/3226870
Cooper, Harry. M.; Lindsay, James J. 1998: “Research synthesis and meta-analysis”. National Conference on Research Synthesis: Social Science Informing Public Policy, Jun, 1994, Washington, DC, US. Sage Publications, Inc.
De Granda-Orive, José Ignacio; Alonso-Arroyo, Adolfo; Roig-Vázquez, Francisco. 2011: “¿Qué base de datos debemos emplear para nuestros análisis bibliográficos? Web of Science versus SCOPUS”. Archivos de Bronconeumología, 47(4), 213. https://doi.org/10.1016/j.arbres.2010.10.007
Del Pino, Rafael; Frías, Antonio; Palomino-Moral, Pedro. 2014: “La revisión sistemática cuantitativa en enfermería”. Revista Iberoamericana de Enfermería Comunitaria: RIdEC, 7(1), 24–40.
Ferreira, Manuel P., Pinto, Claudia F.; Serra, Fernando. 2014. The transaction costs theory in international business research: a bibliometric study over three decades. Scientometrics, 98(3), 1899–1922. https://dx.doi.org/10.1007/s11192-013-1172-8
Franch-Gras, Lluis, Garcia-Roger, Eduardo M.; Serra, Manuel, Carmona María José. 2017: “Adaptation in response to environmental unpredictability”. Proceedings of the Royal Society of London, 284(1868), 20170427. https://doi.org/10.1098/rspb.2017.0427
Freitas, Emmanuela C.; Rocha, Odete. 2011: “Acute and chronic effects of sodium and potassium on the tropical freshwater cladoceran Pseudosida ramose”. Ecotoxicology, 20, 88–93. https://doi.org/10.1007/s10646-010-0559-z
García-Roger, Eduardo M.; Carmona, María José; Serra, Manuel. 2005: “Deterioration patterns in diapausing egg banks of Brachionus (Müller, 1786) rotifer species”. Journal of Experimental Marine Biology and Ecology, 314(2), 149–161. https://doi.org/10.1016/j.jembe.2004.08.023
García-Roger, Eduardo M., Lubzens, Esther; Fontaneto, Diego; Serra, Manuel. 2019: “Facing Adversity, Dormant Embryos in Rotifers”. The Biological Bulletin-US, 237(2), 119–144. https://doi.org/10.1086/705701
Gilbert, Juan Diego; De Vicente, Inmaculada, Ortega, Fernando; Jiménez-Melero, Raquel; Parra, Gema; Guerrero, Francisco. 2015: “A comprehensive evaluation of the crustacean assemblages in southern Iberian Mediterranean wetlands”. Journal of Limnology, 74(1), 169–181. https://doi.org/10.4081/jlimnol.2014.993
Gomes-Barbosa, Luciana; Amorim, Cichelio A.; Parra, Gema, Laco Portinho, Jorge; Morais, Manuela; Morales, Eduardo A.; Menezes, Rosermberg F. 2020: “Advances in limnological research in Earth's drylands”. Inland Waters, 10(4), 429–437. https://doi.org/10.1080/20442041.2020.1728179
Guerrero, Francisco; Parra, Gema; Jiménez-Gómez, Francisco; Salazar, Carlos; Jiménez-Melero, Raquel; Galotti, Anadrea; Ortega, Fernando. 2006: “Ecological studies in Alto Guadalquivir wetlands, a first step towards the application of conservation plans”. Limnetica, 25(1-2), 95–106. https://doi.org/10.23818/limn.25.07
Gutiérrez, María F; Battauz, Yamila; Caisso, Belén. 2017: “Disruption of the hatching dynamics of zooplankton egg banks due to glyphosate application”. Chemosphere, 171, 644–653. https://doi.org/10.1016/j.chemosphere.2016.12.110
Hanson, Mark; Graham, David W.; Babin, Emmanuelle; Azam, Didier; Coutellec, Marie-Agnes; Knapp, Charles W.; Lagadic, Laurent; Caquet, Thierry. 2007: “Influence of isolation on the recovery of pond mesocosms from the application of an insecticide. I. study design and planktonic community responses”. Environmental Toxicology and Chemistry, 26(6), 1265–1279. https://doi.org/10.1897/06-248r.1
Holling, Crawford S. 1973. “Resilience and stability of ecological systems”. Annual Review of Ecology, Evolution, and Systematics, 4, 1–23. https://doi.org/10.1146/annurev.es.04.110173.000245
Kettenring, Karin M.; Adams, Carrie Reinhardt. 2011: “Lessons learned from invasive plant control experiments, a systematic review and meta-analysis”. Journal of Applied Ecology, 48, 970–979. https://doi.org/10.1111/j.1365-2664.2011.01979.x
Koricheva, Julia; Gurevitch, Jessica; Mengersen, Kerrie. (Eds.). 2013: Handbook of meta-analysis in ecology and evolution. New Jersey (USA), Princeton University Press.
Lahr, Joost; Diallo, Alpha. O.; Gadji, Baba; Diouf, Papa S.; Bedaux, Jacques. J. M.; Badji, Aliou; Ndour, Khalifa B.; Andreasen, Jude E.; van Straalen, Nico M. 2000: “Ecological effects of experimental insecticide applications on invertebrates in sahelian temporary ponds”. Environmental Toxicology and Chemistry, 19, 1278–1289. https://doi.org/10.1002/etc.5620190509
Leucht, Stefan; Kissling, Werner; Davis, John M. 2009: “How to read and understand and use systematic reviews and meta-analyses”. Acta Psychiatrica Scandinavica, 119(6), 443–450. https://doi.org/10.1111/j.1600-0447.2009.01388.x.
Lowry, Edward; Rollinson, Emily J.; Laybourn, Adam J.; Scott, Tracy E.; Aiello-Lammens, Mathew E.; Gray, Sarah M.; Mickle, James; Gurevitch, Jessica. 2013: “Biological invasions, a field synopsis, systematic review, and database of the literature”. Ecology and Evolution, 3(1), 182–196. https://doi.org/10.1002/ece3.431
Lupi, Leonardo; Miglioranza, Karina S.; Aparicio, Virginia C.; Marino, Damina; Bedmar, Francisco; Wunderlin, Daniel A. 2015: “Occurrence of glyphosate and AMPA in an agricultural watershed from the southeastern region of Argentina”. Science of the Total Environment, 536, 687–694. https://doi.org/10.1016/j.scitotenv.2015.07.090
Margalef, Ramón (Ed.). 1983: Limnología. Barcelona (España), Omega.
Melaas, Chrsitina. L.; Zimmer, Kyle D.; Butler, Malcolm G.; Hanson, Mark A. 2001: “Effects of rotenone on aquatic invertebrate communities in prairie wetlands”. Hydrobiologia, 459, 177–186. https://doi.org/10.1023/A:1012514124430
Möst, Markus; Chiaia-Hernandez, Aurea C.; Frey, Martin P.; Hollender, Juliane; Spaak, Piet. 2015: “A mixture of environmental organic contaminants in lake sediments affects hatching from Daphnia resting eggs”. Environmental Toxicology and Chemistry, 34, 338–345. https://doi.org/10.1002/etc.2808
Navis, Sabine; Waterkeyn, Aline; Voet, Tom; De Meester, Luc; Brendonck, Luc. 2013: “Pesticide exposure impacts not only hatching of dormant eggs, but also hatchling survival and performance in the water flea Daphnia magna”. Ecotoxicology, 22(5), 803–814. https://doi.org/10.1007/s10646-013-1080-y
Navis, Sabine; Waterkeyn, Aline; Putman, Adinda; De Meester, Luc; Vanermen, Guido; Brendonck, Luc. 2015: “Timing matters, Sensitivity of Daphnia magna dormant eggs to fenoxycarb exposure depends on embryonic developmental stage”. Aquatic Toxicology, 159, 176–183. https://doi.org/10.1016/j.aquatox.2014.12.016
Navis, Sabina; Waterkeyn, Aline; De Meester, Luc; Brendonck, Luc. 2018: “Acute and chronic effects of exposure to the juvenile hormone analog fenoxycarb during sexual reproduction in Daphnia magna”. Ecotoxicology, 27, 627–634. https://doi.org/10.1007/s10646-018-1935-3
Oskina, Natalia; Lopatina, Tatiana; Anishchenko, Olesya; Zadereev, Egor. 2019: “High Resistance of Resting Eggs of Cladoceran Moina macrocopa to the Effect of Heavy Metals”. Bulletin of Environmental Contamination and Toxicology, 102, 335–340. https://doi.org/10.1007/s00128-018-2473-7
Parra, Gema; Jiménez-Melero, Raquel; Guerrero, Francisco. 2005: “Agricultural impacts on Mediterranean wetlands: The effect of pesticides on survival and hatching rates in copepods”. International Journal of Limnology, 41(3), 161–167. https://doi.org/10.1051/limn:20054130161
Pell, Albert; Márquez, Anna; López-Sánchez, José Fermín; Rubio, Roser; Barbero, Mercedes; Stegen, Susana; Queirolo, Fabrizio; Díaz-Palma, Paula. 2013: “Occurrence of arsenic species in algae and freshwater plants of an extreme arid region in northern Chile, the Loa river basin”. Chemosphere, 90(2), 556–564. https://doi.org/10.1016/j.chemosphere.2012.08.028
Portinho, Jorge L.; Nielsen, Daryl L.; Daré, Luana; Henry, Raoul; Oliveira, Regís C.; Branco, Ciro C. 2018: “Mixture of commercial herbicides based on 2,4-D and glyphosate mixture can suppress the emergence of zooplankton from sediments”. Chemosphere, 203, 151–159. https://doi.org/10.1016/j.chemosphere.2018.03.156
Portinho, Jorge. L., Oliveira, Henrique N., Branco, Ciro C. 2021: “Resting egg banks can facilitate recovery of zooplankton communities after short exposure to glyphosate”. Ecotoxicology, 30, 492–501. https://doi.org/10.1007/s10646-021-02371-z
Pranckutė, Raminta. 2021: “Web of Science (WoS) and Scopus: The titans of bibliographic information in today’s academic world”. Publications, 9, 12. https://doi.org/10.3390/publications9010012
Pullin, Andrew S.; Stewart, Gavin B. 2006: “Guidelines for systematic review in conservation and environmental management”. Conservation Biology, 20, 1647–1656. https://doi.org/10.1111/j.1523-1739.2006.00485.x.
Robertson, Bruce A.; Hutto, Richard L. 2006: “A framework for understanding ecological traps and an evaluation of existing evidence”. Ecology, 87(5), 1075–1085. https://doi.org/10.1890/0012-9658(2006)87[1075:affuet]2.0.co;2
Robles-Molina, José; Gilbert-López, Bienvenida; García-Reyes, Juan Francisco; Molina-Díaz, Antonio. 2014: “Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province of Jaén, south east Spain”. Science of the Total Environment, 479, 247–257. https://doi.org/10.1016/j.scitotenv.2014.01.121
Rockström, Johan; Steffen, Will; Noone, Kevin; Persson, Åsa, Chapin, III F. Stuart, Lambin, Eric F.; Lenton, Timothy M.; Scheffer, Marten; Folke, Carl; Schellnhuber, Hans Joachim. 2009: “A safe operating space for humanity”. Nature, 461(7263), 472. https://doi.org/10.1038/461472a
Rogalski, Mary A. 2015: “Tainted resurrection, Metal pollution is linked with reduced hatching and high juvenile mortality in Daphnia egg banks”. Ecology, 96(5), 1166–1173. https://doi.org/10.1890/14-1663.1
Rousseau, Denise M.; Manning, Joshua; Denyer, David. 2008: “Evidence in management and organizational science: Assembling the field’s full weight of scientific knowledge through syntheses”. Academy of Management Annals, 2(2), 475–515. https://doi.org/10.5465/19416520802211651
Scheffer, Marten; Hosper, S. Harry; Meijer, Marie Louise; Moss, Brian; Jeppesen, Erik. 1993: “Alternative equilibria in shallow lakes”. Trends in Ecology and Evolution, 8, 275–279. https://doi.org/10.1016/0169-5347(93)90254-M
Schwartz, Steven S., Jenkins, David G. 2000: “Temporary aquatic habitats, constraints and opportunities”. Aquatic Ecology, 34, 3–8. https://doi.org/10.1023/A:1009944918152
Sievers, Michael; Hale, Robin; Parris, Krsiten M.; Swearer, Stephen E. 2018: “Impacts of human-induced environmental change in wetlands on aquatic animals”. Biological Reviews, 93(1), 529–554. https://doi.org/10.1111/brv.12358
Stampfli, Natalie C.; Knillmann, Saskia; Noskov, Yury A.; Schäfer, Ralph B.; Liess, Mathias; Beketov, Mikhail A. 2014: “Environmental stressors can enhance the development of community tolerance to a toxicant”. Ecotoxicology, 23(9), 1690–1700. https://doi.org/10.1007/s10646-014-1308-5
Stewart, Gavin B.; Pullin, Andrew S.; Coles, Christopher F. 2007: “Poor evidence-base for assessment of wind farm impacts on birds”. Environmental Conservation, 34, 1–11. https://doi.org/10.1017/S0376892907003554
Sutton, Alex J.; Abrams, Keith R.; Jones, David R.; Sheldon, Trevor A.; Song, Fujian. 1998: “Systematic reviews of trials and other studies”. Health Technology Assessment, 2(19), 1–276.
Van den Broeck, Maarten; Waterkeyn, Aline; Rhazi, Laila; Grillas, Patrick; Brendonck, Luc. 2015: “Assessing the ecological integrity of wetlands - indicators applicable for temporary wetlands”. Ecological Indicators, 54, 1–11. https://doi.org/10.1016/j.ecolind.2015.02.016
Vandekerkhove, Jochen; Declerck, Steven; Brendonck, Luc; Conde-Porcuna, José María; Jeppesen, Erik; Johansson, Lisselotte S., De Meester, Luc. 2005: “Uncovering hidden species, hatching diapausing eggs for the analysis of cladoceran species richness”. Limnology and oceanography, 3(9), 399–407. https://doi.org/10.4319/LOM.2005.3.399
Wetzel, Robert G. (Ed). 2001: Limnology, Lake and river ecosystems. Academic Press.
Williams, William. D. 1999: “Conservation of wetlands in drylands, a key global issue”. Aquatic Conservation. Marine and Freshwater Ecosystems, 9(6), 517–522. https://doi.org/10.1002/(SICI)1099-0755(199911/12)9:6<517::AID-AQC383>3.0.CO;2-C
Williams, Penny; Biggs, Jeremy; Fox, Gill; Nicolet, Pascale; Whitfield, Mericia. 2001: “History, origins and importance of temporary ponds”. Freshwater Forum, 17, 7–15.
_______________________________
1 Margalef, 1983. Williams et al., 2001.
2 Schwartz; Jenkins, 2000. Gilbert et al., 2015.
4 Brendonck; De Meester, 2003.
5 Wetzel, 2001. Rogalski, 2015.
6 Brendonck; De Meester, 2003. Vandekerkhove et al., 2005. Franch-Gras et al., 2017.
8 Van den Broeck et al., 2015.
9 Angeler; García, 2005. García-Roger; Carmona; Serra, 2005. Van den Broeck et al., 2015.
11 Angeler; García, 2005. Angeler, 2007.
14 Robles-Molina et al., 2014. Lupi et al., 2015.
16 Parra; Jiménez-Melero; Guerrero, 2005. Gutiérrez; Battauz; Caisso, 2017.
17 Angeler, 2007. Pell et al., 2013.
18 Gomes-Barbosa et al., 2020.
21 Holling, 1973. Allen et al., 2019.
22 Scheffer et al., 1993. Ansari et al., 2011.
24 Angeler; Moreno, 2006; 2007.
26 Kettenring; Adams, 2011. Lowry et al., 2013. Pullin; Stewart, 2006. Stewart; Pullin; Coles, 2007.
27 Koricheva; Gurevitch; Mengersen, 2013.
29 Burnham, 2006. De Granda-Orive; Alonso-Arroyo; Roig-Vázquez, 2011. Pranckutė, 2021.
32 Gutiérrez; Battauz; Caisso, 2017.
33 Angeler; García, 2005. Van den Broeck et al., 2015.
34 Aránguiz-Acuña et al., 2018.
36 Möst et al., 2015. Portinho et al., 2018. Portinho; Oliveira; Branco, 2021.
37 Aránguiz-Acuña; Pérez-Portilla, 2017. Navis et al., 2013; 2015.
38 Gutiérrez; Battauz; Caisso, 2017.
40 Portinho; Oliveira; Branco, 2021.
42 Angeler; García, 2005. Aránguiz-Acuña; Serra, 2016. Rogalski, 2015.
43 Portinho; Oliveira; Branco, 2021.
47 Angeler, 2021, 11.
49 Brendonck; De Meester, 2003. Cáceres, 1997.
54 Gutiérrez; Battauz; Caisso, 2017.
56 Ferreira; Pinto; Serra, 2014.
57 Rousseau; Manning; Denyer, 2008.